What Name Is Given To Substances That Resist Changes In Ph
14.10: Buffers- Solutions That Resist pH Change
- Page ID
- 98107
Learning Objective
- Ascertain buffer and draw how information technology reacts with an acrid or a base.
Weak acids are relatively common, fifty-fifty in the foods we consume. But nosotros occasionally come across a strong acid or base, such as tum acid, that has a strongly acidic pH of 1–two. By definition, strong acids and bases can produce a relatively large corporeality of hydrogen or hydroxide ions and, equally a upshot, accept marked chemic activity. In add-on, very small-scale amounts of strong acids and bases tin can alter the pH of a solution very apace. If i mL of tum acid [which we will approximate equally 0.05 G HCl(aq)] is added to the bloodstream, and if no correcting machinery is present, the pH of the blood would go from about 7.4 to most 4.nine—a pH that is not conducive to life. Fortunately, the body has a machinery for minimizing such dramatic pH changes.
This machinery involves a buffer, a solution that resists dramatic changes in pH. Buffers exercise so past being equanimous of certain pairs of solutes: either a weak acid plus a salt derived from that weak acrid, or a weak base plus a table salt of that weak base of operations. For example, a buffer tin be composed of dissolved acetic acid (HC2H3Otwo, a weak acid) and sodium acetate (NaC2H3O2, a salt derived from that acid). Another example of a buffer is a solution containing ammonia (NH3, a weak base) and ammonium chloride (NHfourCl, a common salt derived from that base).
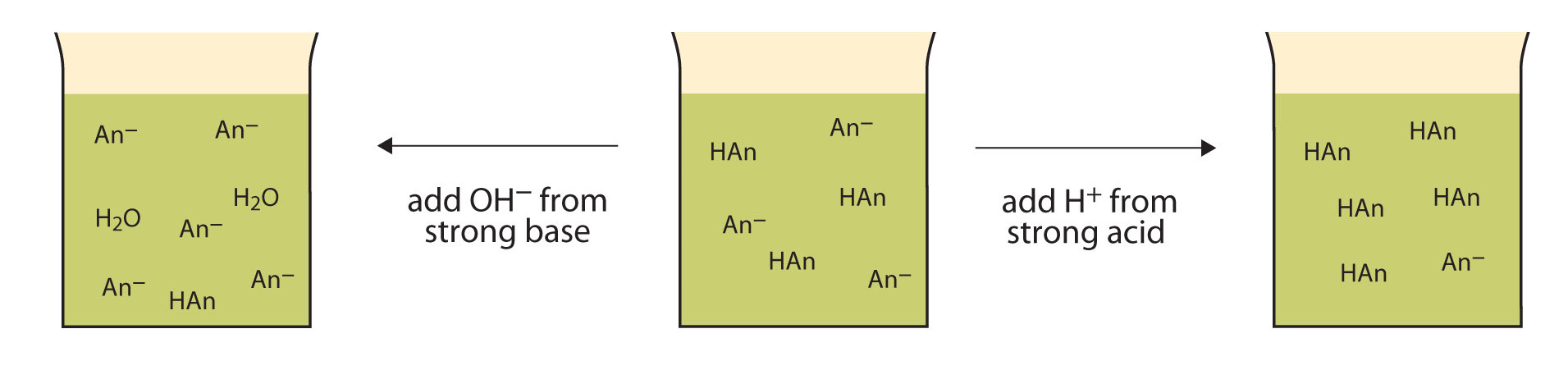
Let usa apply an acetic acid–sodium acetate buffer to demonstrate how buffers piece of work. If a potent base—a source of \(\ce{OH^{-}(aq)}\) ions—is added to the buffer solution, those hydroxide ions will react with the acerb acid in an acid-base reaction:
\[\ce{HC2H3O2(aq) + OH^{-}(aq) \rightarrow H2O(ℓ) + C2H3O^{-}2(aq)} \characterization{Eq1}\]
Rather than irresolute the pH dramatically by making the solution basic, the added hydroxide ions react to make water, and the pH does not change much.
Many people are aware of the concept of buffers from buffered aspirin, which is aspirin that also has magnesium carbonate, calcium carbonate, magnesium oxide, or another salt. The table salt acts like a base, while aspirin is itself a weak acid.
If a strong acid—a source of H+ ions—is added to the buffer solution, the H+ ions will react with the anion from the common salt. Because HCiiH3O2 is a weak acid, information technology is not ionized much. This ways that if lots of hydrogen ions and acetate ions (from sodium acetate) are present in the same solution, they will come together to make acerb acid:
\[\ce{H^{+}(aq) + C2H3O^{−}two(aq) \rightarrow HC2H3O2(aq)} \characterization{Eq2}\]
Rather than changing the pH dramatically and making the solution acidic, the added hydrogen ions react to make molecules of a weak acid. Figure \(\PageIndex{i}\) illustrates both actions of a buffer.
Buffers made from weak bases and salts of weak bases act similarly. For example, in a buffer containing NHiii and NHfourCl, ammonia molecules can react with whatever excess hydrogen ions introduced by potent acids:
\[\ce{NH3(aq) + H^{+}(aq) \rightarrow NH^{+}4(aq)} \label{Eq3}\]
while the ammonium ion (\(\ce{NH4^{+}(aq)}\)) can react with any hydroxide ions introduced by strong bases:
\[\ce{NH^{+}4(aq) + OH^{-}(aq) \rightarrow NH3(aq) + H2O(ℓ)} \label{Eq4}\]
Example \(\PageIndex{one}\): Making Buffer Solutions
Which solute combinations can make a buffer solution? Assume that all are aqueous solutions.
- HCHOii and NaCHOii
- HCl and NaCl
- CH3NH2 and CH3NH3Cl
- NH3 and NaOH
Solution
- Formic acid (HCHO2) is a weak acrid, while NaCHO2 is the salt made from the anion of the weak acid—the formate ion (CHO2 −). The combination of these two solutes would make a buffer solution.
- Hydrochloric acid (HCl) is a strong acrid, not a weak acid, so the combination of these two solutes would not make a buffer solution.
- Methylamine (CH3NH2) is similar ammonia with i of its hydrogen atoms substituted with a CHthree (methyl) group. Because it is not on our list of stiff bases, we tin assume that it is a weak base. The compound CH3NH3Cl is a salt made from that weak base, so the combination of these two solutes would make a buffer solution.
- Ammonia (NH3) is a weak base, but NaOH is a stiff base. The combination of these 2 solutes would non make a buffer solution.
Exercise \(\PageIndex{1}\)
Which solute combinations can brand a buffer solution? Assume that all are aqueous solutions.
- NaHCO3 and NaCl
- H3PO4 and NaH2PO4
- NHiii and (NHfour)3POiv
- NaOH and NaCl
- Respond a
- Yes.
- Answer b
- No. Need a weak acid or base and a salt of its cohabit base of operations or acrid.
- Answer c
- Yes.
- Answer d
- No. Need a weak base of operations or acrid.
Buffers work well only for limited amounts of added strong acid or base. In one case either solute is all reacted, the solution is no longer a buffer, and rapid changes in pH may occur. We say that a buffer has a certain chapters . Buffers that have more than solute dissolved in them to outset with accept larger capacities, as might be expected.
Human blood has a buffering organisation to minimize farthermost changes in pH. One buffer in blood is based on the presence of HCOthree − and H2COiii [H2COiii is another fashion to write COii(aq)]. With this buffer nowadays, fifty-fifty if some stomach acid were to find its way directly into the bloodstream, the change in the pH of claret would be minimal. Inside many of the body'southward cells, there is a buffering system based on phosphate ions.
Career Focus: Blood Bank Engineering Specialist
At this point in this text, you should have the thought that the chemistry of blood is fairly circuitous. Because of this, people who piece of work with blood must be especially trained to work with information technology properly.
A blood bank technology specialist is trained to perform routine and special tests on blood samples from claret banks or transfusion centers. This specialist measures the pH of blood, types it (co-ordinate to the claret'due south ABO+/− blazon, Rh factors, and other typing schemes), tests information technology for the presence or absence of various diseases, and uses the blood to decide if a patient has any of several medical problems, such equally anemia. A blood bank technology specialist may also interview and prepare donors to give blood and may actually collect the claret donation.
Blood bank technology specialists are well trained. Typically, they require a college degree with at least a twelvemonth of special grooming in blood biology and chemistry. In the United States, grooming must conform to standards established by the American Clan of Blood Banks.
Key Takeaway
- A buffer is a solution that resists sudden changes in pH.
Contributions & Attributions
This folio was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
-
Marisa Alviar-Agnew (Sacramento City Higher)
-
Henry Agnew (UC Davis)
Source: https://chem.libretexts.org/Courses/College_of_Marin/CHEM_114:_Introductory_Chemistry/14:_Acids_and_Bases/14.10:_Buffers-_Solutions_That_Resist_pH_Change
Posted by: hernandezantionne.blogspot.com

0 Response to "What Name Is Given To Substances That Resist Changes In Ph"
Post a Comment